Welcome to ATMP Sweden!
ATMP Sweden is the national network of Sweden’s activities within medicines based on genes, cells or tissue engineering, classified as Advanced Therapy Medicinal Products (ATMPs) in Europe. Our goal is to promote the collaboration and communication needed for accelerated, effective ATMP based patient solutions.

News
2025-03-20
New EU Clinical Trial Map Enhances Patient Access to Research
The European Medicines Agency (EMA) has launched a Clinical Trial Map, helping patients and healthcare professionals across the EU easily find ongoing clinical trials and improve access to ATMPs.
Read article
2025-03-20
Unregulated ATMPs in the EU: A Threat to Patient Safety
Unregulated ATMPs are being marketed in the EU, posing serious health risks. Authorities urge patients to verify treatments and seek only approved therapies.
Read article
2025-02-24
ATMP Center Joins EU-Funded Project to Enhance Cell Therapy Manufacturing
The ATMP Center at Skåne University Hospital in Lund is joining a European initiative to improve the efficiency and sustainability of manufacturing processes for cell and gene therapies.
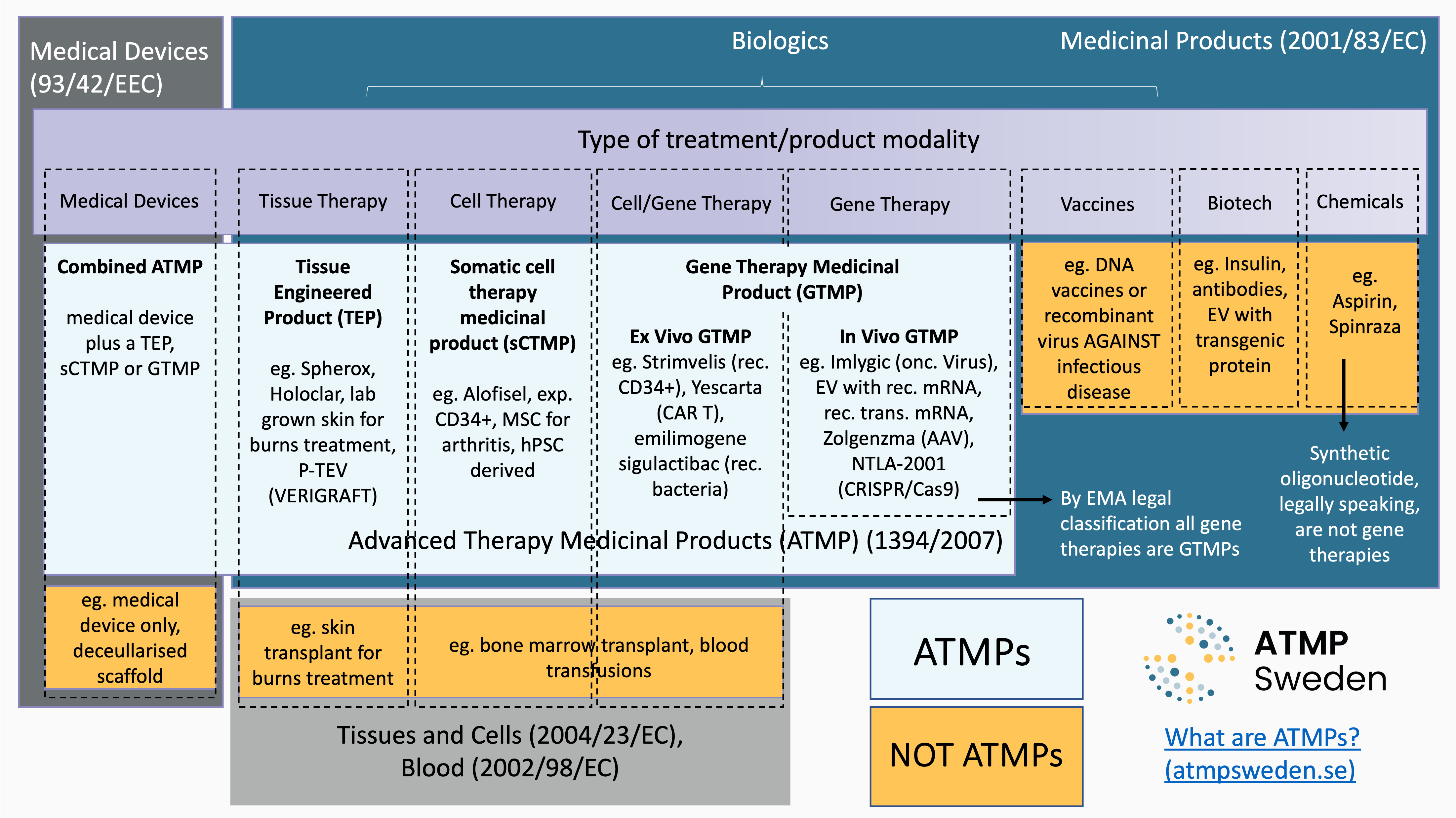
Read articleWhat are ATMPs?
Conferences & events
Conference
Mötesplats ATMP i Väst 2025
07 May
Sahlgrenska Universitetssjukhuset
Organizer: Lif - de forskande läkemedelsföretagen & Västra Götalandsregionen
Conference
PDA Virus Conference 2025
24 June
Scandic Gothenburg Central, Gothenburg, Sweden.
Organizer: PDA Europe
See you in Gothenburg, Sweden!
Read moreConference
PDA ATMPs Conference 2025
26 June
Scandic Gothenburg Central, Gothenburg, Sweden
Organizer: PDA Europe
See you in Gothenburg, Sweden!
Read moreResources

Learn more about the Health Technology Assessment related issues of your ATMP development.

Support in navigating ATMP development needs prompting the right competence, need and time.

Find or advertise ATMP relevant job opportunities.
