Welcome to ATMP Sweden!
ATMP Sweden is the national network of Sweden’s activities within medicines based on genes, cells or tissue engineering, classified as Advanced Therapy Medicinal Products (ATMPs) in Europe. Our goal is to promote the collaboration and communication needed for accelerated, effective ATMP based patient solutions.

News

2024-10-21
We say farewell to our dearest Jukka
Our colleague, friend and work husband, taken too soon
Read article
2024-09-23
ATMP2030 releases annual ATMP report for 2024
A booming Swedish ecosystem from research to SME, clinical treatment and employment
Read article
2024-09-02
Uppdatering om Nationell Samordningsfunktion kring ATMP
Kristina Levan är utsedd till ordförande för den samordningsgrupp och Ewa Ellis som koordinator i projektet
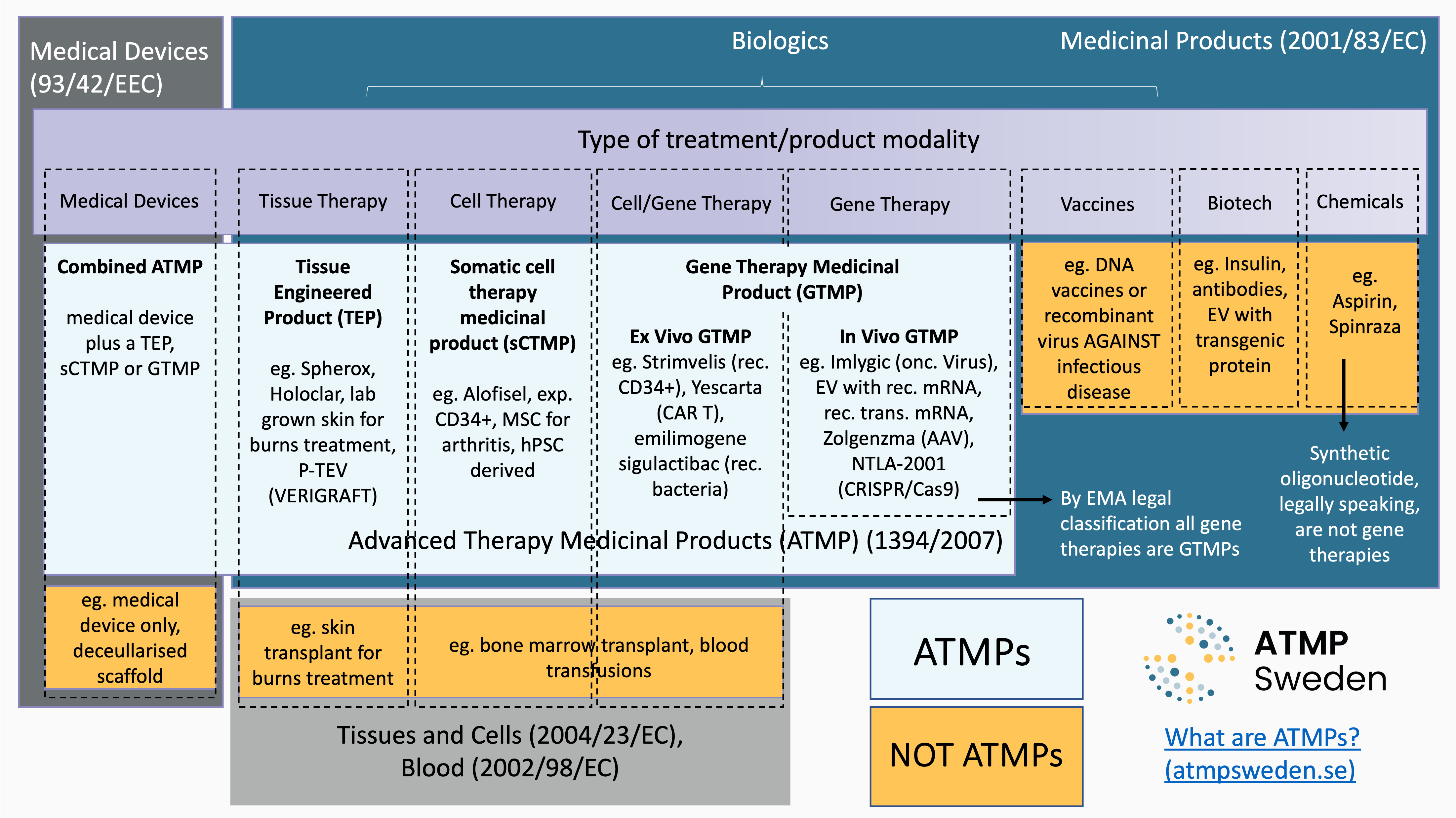
Read articleWhat are ATMPs?
Conferences & events
Seminar
Presentation of Swedish annual ATMP report
25 October
Online - ZOOM
Organizer: ATMP2030 and Apotekarsocieteten
Learn more about the latest developments in the Swedish ATMP landscape!
Read moreConference
BIO-Europe
Course
Introduction to ATMPs, 1.5 ECTS
18 November
Online
Organizer: National ATMP Research School
A course offered by the National ATMP Research School
Read moreResources

Learn more about the Health Technology Assessment related issues of your ATMP development.

This guide will provide useful information and insights throughout the ATMP development process.

Find or advertise ATMP relevant job opportunities.
